Mayo Clinic and LisenID Partner to Revolutionize Early Cancer Detection
Summary
Full Article
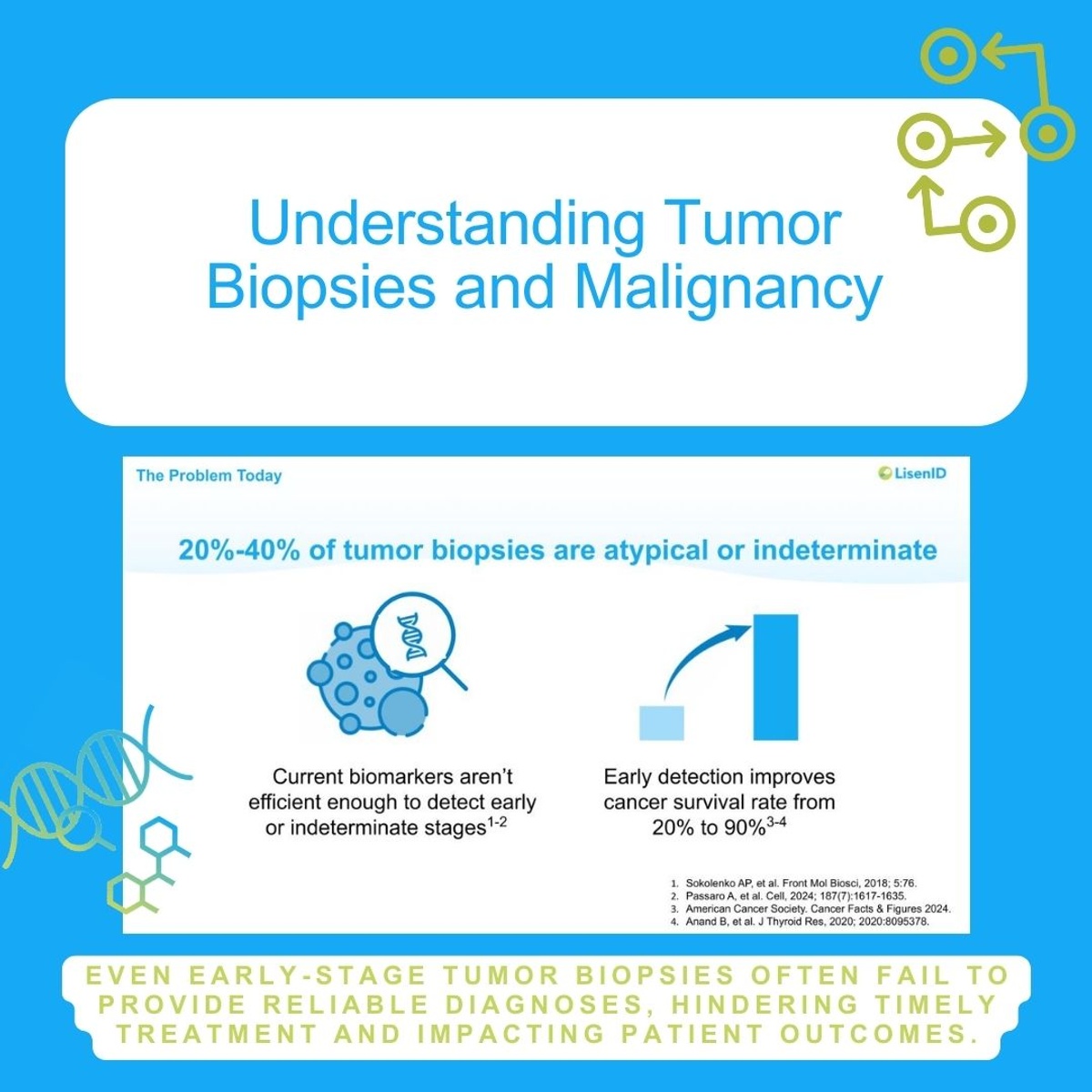
LisenID, a diagnostic technology company, has partnered with Mayo Clinic to develop groundbreaking early cancer detection methods using a novel approach called Quantitative Chromogenic Imprinted Gene In-Situ Hybridization (QCIGISH). The collaboration aims to create more accurate diagnostic tools, initially focusing on lung cancer detection through advanced biomarker identification.
The joint research initiative targets peripheral pulmonary lesions, an area traditionally challenging for precise diagnostics. By leveraging QCIGISH technology, which can detect cancer at its inception through epigenetic imprinting biomarker abnormalities, the partnership seeks to provide more sensitive and specific cancer screening methods.
Previous research by LisenID demonstrates impressive diagnostic capabilities, with over 95% sensitivity and 90% specificity for detecting early-stage cancers across multiple types, including lung, thyroid, cervical, and other malignancies. This collaboration represents a significant advancement in cancer diagnostics, potentially enabling earlier intervention and improving patient prognosis.
The initial project will develop a laboratory-developed test (LDT) for lung cancer detection using transbronchial needle aspiration samples. If successful, this technology could revolutionize how medical professionals identify and respond to early-stage cancers, offering a more objective and clinically actionable diagnostic approach.
By combining LisenID's innovative epigenetic imprinting technology with Mayo Clinic's extensive clinical research expertise, this collaboration has the potential to transform cancer diagnostics, providing hope for more effective early detection and treatment strategies.

This story is based on an article that was registered on the blockchain. The original source content used for this article is located at Reportable
Article Control ID: 50952