HeartBeam Inc. Revolutionizes Cardiac Care with FDA-Cleared Portable ECG System
Summary
Full Article
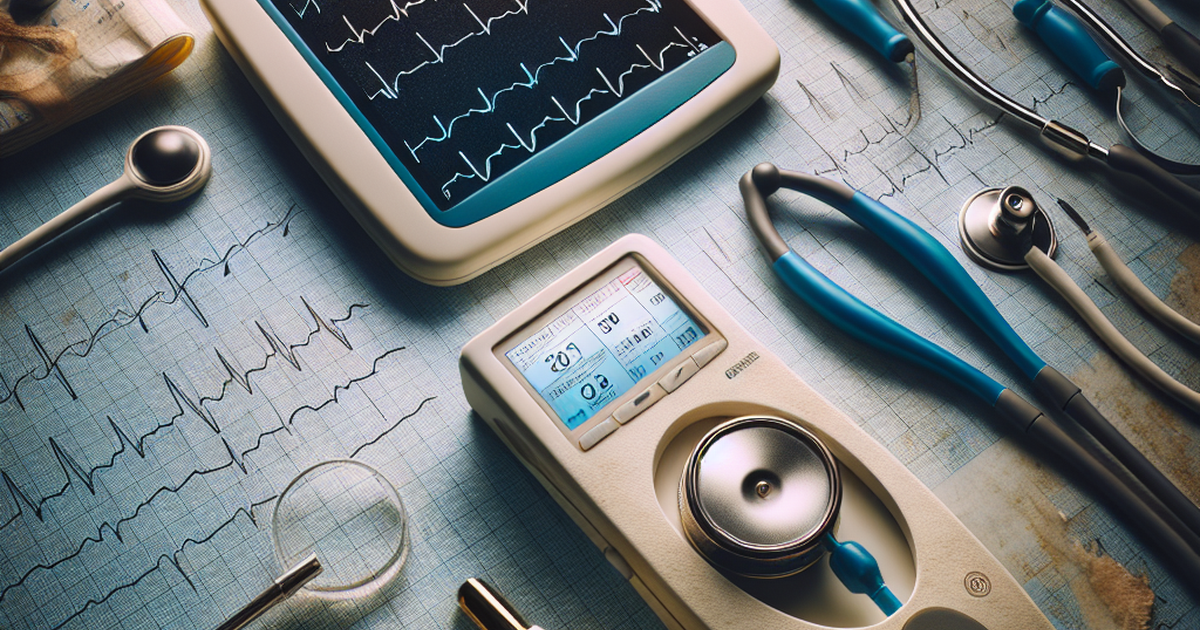
HeartBeam Inc. (NASDAQ: BEAT) has achieved a significant milestone in cardiac care technology with the FDA clearance of its innovative HeartBeam system. This credit card-sized, fully portable device is designed to capture the heart's electrical activity from three distinct directions, offering a breakthrough in ambulatory cardiac care. The system enables patients and physicians to access critical arrhythmia data outside the confines of traditional clinical settings, representing a pivotal advancement in the field.
The HeartBeam system is equipped with five electrodes and records three-directional electrical signals over a 30-second period. Its portability and ease of use make it the first of its kind to receive FDA clearance, setting a new standard for cardiac monitoring devices. This development not only enhances patient care by facilitating early detection and management of arrhythmias but also underscores the potential for future innovations in synthesized 12-lead ECG generation.
The implications of this technology extend beyond immediate clinical benefits. By enabling arrhythmia assessment in non-traditional settings, the HeartBeam system could significantly reduce healthcare costs associated with hospital visits and prolonged monitoring. Furthermore, its portability opens up new possibilities for remote patient monitoring, potentially transforming the landscape of cardiac care.
For more information on HeartBeam Inc. and its groundbreaking technology, visit https://ibn.fm/0TAG4. Investors seeking the latest updates on BEAT can find comprehensive coverage in the company's newsroom at https://ibn.fm/BEAT.

This story is based on an article that was registered on the blockchain. The original source content used for this article is located at InvestorBrandNetwork (IBN)
Article Control ID: 138327